THE BODY’S GUT MICROBIOME: Our Vitality Organ (16 March 2022)
Scott Emerson, MD, ABIHM
Timelesshealing
Definitions (1, 2, 3, 4, 5, 16)

- Prebiotics are foods or substrates, that are selectively utilized by host microorganisms conferring a health benefit to the host. These are mainly plant based soluble fiber carbohydrates and plant derived proteins found abundantly in plant forward, Mediterranean style, and vegetarian diets. Proper feeding with prebiotics acts to increase the survival, numbers and diversity of beneficial bacteria. Some common prebiotic food sources are onions, beans, legumes, oats, asparagus, garlic, broccoli, cauliflower, apples, kiwi fruit, artichokes, and bananas.
- Probiotics are live microorganisms that when administered in adequate amounts, confer a health benefit on the host. These benefits include preventing leaky gut, regulating our immune system, crowding out and killing harmful bacteria, and extraction, synthesis and absorption of many nutrients and metabolites for our benefit (bile acids, amino acids, vitamins, and short chain fatty acids)
- Postbiotics are a preparation of inanimate microorganisms and (or) their components, with or without microbial metabolites, which confers a safe health benefit on the host. A partial list would include: vitamin B12 & folate, tryptophan amino acid, lipopolysaccharide fragments of bacterial cell walls, bacterial enzymes, gamma amino butyric acid neurotransmitter, and short chain fatty acid bacterial metabolites (acetate, propionate, butyrate).
- Gut microbiota is the total of all microbial species and types of organisms living within our GI tract. This includes bacteria, viruses, fungi, and protozoa. The bacterial fraction is the most studied. There is an average of 40 trillion bacteria living predominantly in our colon bioreactor – the final segment of our GI tract.
- Gut Microbiome refers to the entire catalogue of these microbes and their genetic contribution to our own human genes. This 3.3 million-gene contribution of non-human, non-redundant bacterial genes is quite impressive and outnumbers our 21 thousand human genes by 150 times. There is much more of their stuff in your body than there is your stuff.
- Dysbiosis is characterized as a disruption to the microbiota homeostasis by an imbalance in the microflora, changes in their functional composition and metabolic activities, or a shift in their local distribution.
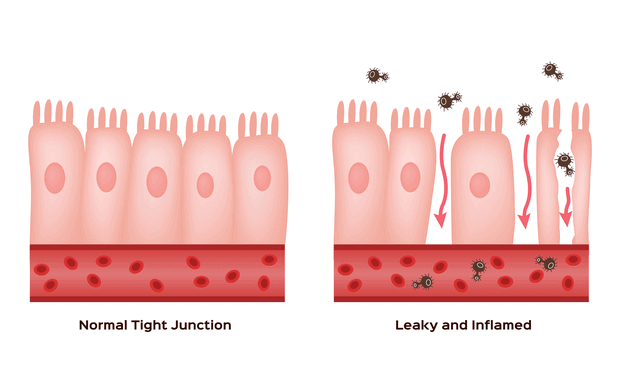
- “Leaky Gut” syndrome is a symptom complex that includes: abdominal bloating, cramps, constipation, diarrhea, food sensitivities, joint and muscle pains, chronic fatigue, brain fog, mood swings, acne and skin rashes. It is commonly associated with obesity and elevated blood cholesterol and glucose and the Standard American Diet. Dysbiosis created by poor dietary choices, stress, environmental toxins, lack of exercise, or genetic susceptibility seems to be the most common root causes. This then leads to a disruption in the normal barrier gate and fence, tight junction function of the intestinal epithelial cells that allows bacteria, bacterial fragments, and food particles to enter the blood stream and create a chronic generalized inflammatory reaction and a “metabolic endotoxemia”. Improving diet and intermittent fasting are the primary interventions for this condition.
What Appears Certain. (1, 2, 6 7, 8, 9, 10, 22)
- Many Uncertainties and Mysteries Remain. The gut microbiome is an amazingly complex, dynamic and powerful generator and regulator of our health and well being. Our knowledge of its actions and effects is rapidly expanding and changing, but we are just beginning to scratch the surface.
- The Gut Microbiome Is the Newly Discovered Organ In Your Body. Your microbiome exists within you as a “surperorganism” of different bacterial species interfacing, directing and communicating with each other, as well as with our bodies via intimate direct relationships with our intestinal epithelial cells, gut associated lymphatic tissue, and the intestinal nervous system. It exerts indirect effects as well on our organs distant from the intestinal tract. This includes the brain, liver, heart, lung, pancreas and skin.

- Your Mom’s Great Gift is Your Core Microbiome. Your “core” microbiome is ideally acquired via vaginal birth and breast-feeding from your mom, which offers the infant the best quality and most diversity of microbiota. This “core” remains remarkably stable throughout your life but can have its diversity significantly degraded due to choosing the Standard American Diet over a Plant Forward Based Diet (see Culinary Medicine Recipes post), or lack of exercise, or repeated or prolonged antibiotic use.
- Diversity is Strong & Healthy / Low Diversity is Weak and Sick. The more diverse the number of species that make up your particular microbiota, the more robust and resistant to diseases you are, and healthier you will be. Lack of microbiota diversity has been well documented to increase the instability of the microbiome and decreases its capacity to return to “core” equilibrium states after environmental stresses and disruptions like antibiotic use. This lack of diversity creates a tendency toward “dysbiosis”: a persistent imbalance in the patterns of the gut microbiota community ecosystems that is strongly correlated with diseases like obesity, irritable bowel syndrome, inflammatory bowel disease, diabetes, cardiovascular disease, central nervous system disorders, and cancer incidence and it’s relapse post treatment. Yes, that’s almost any disease you can think of. Lifestyle choices have a major influence on diversity of your microbiota with plant based diets and especially regular exercise dramatically improving diversity of your gut microbiome.
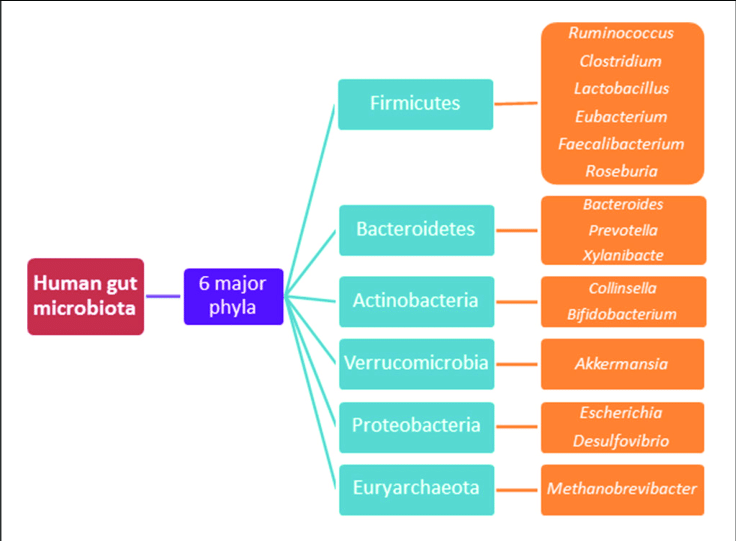
- Increased Firmicutes/Bacteroidetes Phylum Ratios Signal Dysbiosis. There are over 30,000 different species of bacteria currently identified, grouped into 90 different phyla (the largest divisions of related bacteria). Only 6 of these phyla are known to make up almost all of our total gut microbiota. And, only 2 of these phyla, Firmicutes with 200 different species, and Bacteroidetes with 7000 different species, represent 90 to 95 % of all the human gut microbiota. Obese individuals in addition to overall decrease in bacterial diversity, have an increase in the ratio of these Firmicutes to Bacteroidetes phyla in their gut. The healthy non-obese ratio appears to be 80% Firmicutes to 15% Bacteroidetes. However, this ratio must still be considered a very coarse measure considering the complexity of the gut microbiome and our current knowledge. The Standard American Diet (animal protein forward, high in refined sugar & starch, low in fiber) has a dramatic and rapid effect on increasing this Firmicutes to Bacteroidetes ratio into an unhealthy skew.
- Gut Dysbiosis Has a Direct Association With COVID-19 Infection and Its Severity. Obesity is now a well documented risk factor for severe COVID-19 and death. Now there is overwhelming evidence that an underlying gut dysbiosis is also directly associated with COVID-19 infections, and the likelihood of progression to severe COVID-19. The less diversity there is in the gut microbiota, the greater the risk. The possibility of dysbiosis playing an important role in the development of “long COVID” is currently under investigation.
- Reductionist Treatments of “Dysbiosis” are Premature at this Time. The uses of specific individual species of a targeted probiotic, like non-pathogenic Clostridia sp. and others, without a better understanding of the extreme complexity of the gut microbiome, cannot be condoned. These bacterial microbes, a community of 40 trillion organisms containing over 1000 different species, are in constant biochemical crosstalk with with our bodies. This extreme degree of complexity dictates that each person’s “dysbiosis” may be a little different. However, broad, well documented treatments like, exercise, a good prebiotic diet, stress reduction, and pulsed broad spectrum probiotics to minimize collateral damage from antibiotics, should be the initial steps in correcting any “dysbiosis”. This will direct each specific individual’s core microbia to naturally regain balance, and is the safest, likely most effective, sustainable, and least expensive first intervention currently available.
FOR 5 STEPS YOU CAN TAKE TO CREATE AND MAINTAIN THE VITAL MICROBIOME ORGAN WITHIN YOU, SCROLL TO THE LAST SECTION OF THIS POST
The You Part of the Relationship: The Direct Interface (7, 8, 9,10, 11)
The intestine is the largest immune organ in the body and is the most susceptible site for invasive bacteria and viruses. Despite direct exposure to a large number of microorganisms and foreign antigens, a unique intestinal immune system and barrier function maintains balance of the GI tract and the entire body. The inner lining of our intestine is covered with a variety of epithelial cells with differing specialized and coordinated immune and barrier functions in direct interface with the gut microbiome. These epithelial cells, and especially the gut associated lymphoid cells and dendritic immune cells, have pattern recognition receptors on their surface that sense microbe associated molecular patterns (MAMPs) and continually monitor the quantity and quality of the gut microbiome.
The Goblet cells produce and secrete mucus – a hydrated gel consisting of the protein mucin, that is massively coated with long chain sugar molecules. This mucus coats the inner lining of intestinal epithelial cells forming a thick physical barrier between them and the outside world contents of the intestinal lumen. This mucus defends against pathogenic bacteria, as well as providing a “safe harbor” for friendly gut microbes. Mucin production by Goblet cells has recently been shown to be regulated by prebiotics and their specific effects on two different species of friendly bacteria (probiotics) inhabiting the gut microbiome.


Whole apples contain complex pectin prebiotics that feed Bacteroides thethaiotaomicron, a species that can signal an increase in expression of the genes for mucin production within Goblet cells. On the other hand, Kiwi fruit contains prebiotic compounds that increase the abundance of Faecalibacterium prausnitzii that signals a decrease in the expression of genes for mucin production by Goblet cells. This is just one specific example of how foods you eat (prebiotics), by affecting the growth of different bacteria (probiotics) to produce nuclear transcription molecules (postbiotics) targeting Goblet cells (the you part), end up regulating the amount of mucus you have in your intestine at any given time.
The Paneth cells, another specialized epithelial cell of the intestinal lining, produce Host Defense Peptides (HDPs) – short chains of positively charged amino acids that act as outreach or effector molecules of our intestinal immune system. The specific types of HDPs secreted act to fine tune and regulate the various numbers and types of bacterial species occupying the gut microbiome at any given time. There are complex multiple different actions possible with each different HDP based on their conformational state (folded or linear) or their active cleaved degradation products. Thus, there may be an amazing array of unique HDPs possible, each with specific targets and activities. HDPs are directed out into the mucus layer as endogenously produced antibiotics where they can kill pathogenic bacterial by disrupting their cell membranes. There, they can also block attachment of bacteria to each other thus breaking up biofilm colonies of specific pathogenic bacteria. They also can block pathogenic bacteria from binding to intestinal epithelial cells. Friendly bacteria, if they are sensed as over represented or out of balance for optimal functioning of the intestinal cells, may also become targets for HDPs. Sometimes the HDPs simply stimulate the Goblet cells to produce more mucus. The signaling cascade for the HDPs flows from the bacterial produced MAMPs activating receptors on lymphoid cells that are constantly scanning for specific molecular patterns or metabolites of different bacteria. Once activated, they respond by producing and releasing down stream molecular messengers like cytokines. The cytokines and other molecules are sensed by the Paneth cells that then manufacture and release HDPs into the local environment for modification of bacterial activity occurring nearby.
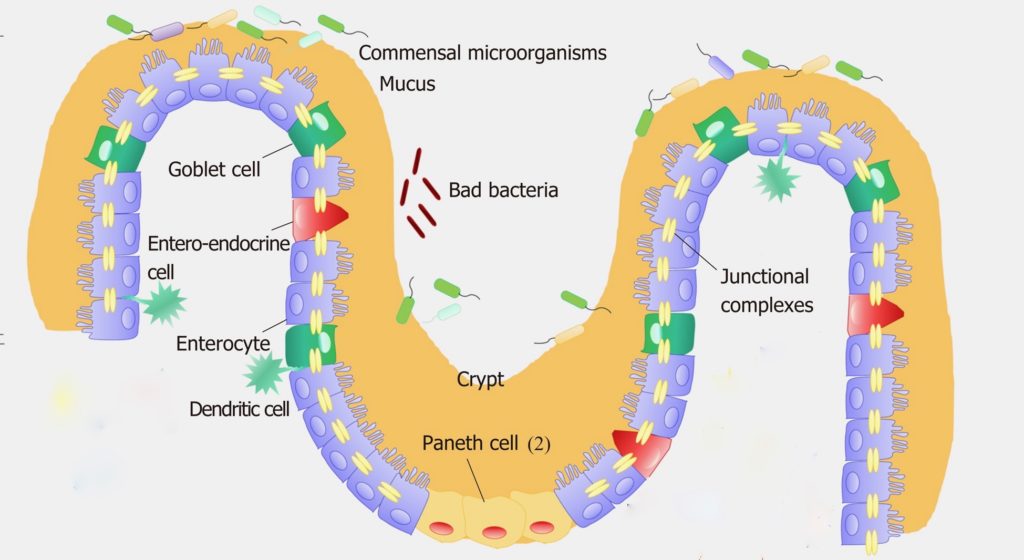
The Enteroendocrine cells (EECs) of the intestinal lining are the sensory sentinels of the intestinal epithelium, responding to nutrients within the intestinal conduit by secreting peptide hormones and cytokines creating a wide array of regulatory responses. A partial list would include gastrointestinal enzyme secretion and hormone release, strengthening tight junctions of intestinal epithelial cells, and prompting release of secretory IgAs from the gut associated lymphoid tissue into the mucus layer. Additionally, they also influence Goblet and Paneth cell function, coordinate gut motility as well as appetite regulation. They actively monitor bacterial postbiotic metabolites like peptides and SCFAs and bacterial toxins, providing local regulation of nutrient absorption and expulsion of bacterial toxins from other intestinal epithelial cells. EECs are flexible, dynamic, relatively long lived, and are very impressionable and sensitive to environmental influences like postbiotics from the gut microbiota. Changes in bacterial postbiotic metabolites can interact with EECs to disorganize tight junctions of intestinal epithelium creating leaky gut and metabolic syndrome. The exact mechanisms of this are poorly understood.
Recently, a species of bacteria of the Veillonella genus was found to be elevated in the gut microbiome of those who exercised regularly, and had a normal BMI. This bacterium was missing in obese individuals. Veillonella generates propionate as a postbiotic, which is sensed by the EECs resulting in the release of PYY, and glucagon like peptide – 1 (GLP-1) hormones. Both of these peptides create loss of appetite sensation (decreased the I’m hungry hormone, ghrelin, secretion) and decreased food intake. PYY is found to be markedly decreased in obese persons. This is just one example of how, in a very complex signaling chain, a prebiotic (in this case exercise) can increase a probiotic species (Veillonella) and its postbiotic metabolites (short chain fatty acids) to interact with a specific specialized type of intestinal epithelial cell (the EEC in the colon), to release its messenger peptide hormones to a distant organ (the stomach), to regulate the release of another peptide messenger hormone (ghrelin), to even more distant organs (the brain). Multiply this times many thousands of other as yet unknown specific pathways certain to exist, and you can begin to imagine the exceedingly complex and vast influence that the gut microbiome has within your body.
The Them Part of the Relationship: Who They Are / How They Live (2, 5, 6, 7, 14, 15, 17, 18, 19, 21)
Probiotics, Who Are the Healthy Gut Microbiota? Unfortunately to date, based largely on population studies, we have only a rough and broad idea. Two major bacterial phyla make up 90 plus percent of all human gut microbiota. These are the FIRMICUTES and BACTEROIEDETES phyla. There are many other minor species of bacteria within each phylum not listed below, that may play significant modifying roles in overall health with more being discovered all the time.
1. FIRMICUTES. Prominent species within these phyla are: Lactobacillus sp., Ruminococcus sp., Faecalibacterium sp., Enterococcus sp. and Clostridium sp. It is important to note that not all Clostridium species, dominant within the FIRMICUTES phylum of bacteria, are harmful like Clostridium difficile & Clostridium botulinum are.
- Clostridia sp. This genus is divided into multiple clusters, with clusters # 4 (with 4 species) and # 14a (with 21 species) dominating and representing up to 40 percent of all the bacteria in the gut microbiome. We all carry much smaller numbers of pathogenic Clostridia sp. like C. difficile that are constantly held in check by the larger numbers on the beneficial Clostridia clusters. These beneficial Clostridia thrive on fermentation of soluble plant fiber and are the primary producers of butyric acid, a highly beneficial postbiotic for us. In addition, one member of Clostridial cluster #4, Faecalibacterium prausnitzii, has been shown to exert powerful immune modulating effects with the host, improving gut immune tolerance, and preventing over reactivity by the gut associated lymphoid tissue.
- Lactobacillus sp. There are over 50 species. They act to produce lactic acid from plant-based carbohydrates. They also produce bacteriocidins that have demonstrated antagonistic activity against harmful bacteria like pathogenic E. coli and Salmonella sp. This genus possesses an enhanced ability to attach and colonize the gut mucosal environment, and strongly influences the existing microbial population in favor of your health.
2. BACTEROIDETES. Prominent species within this phyla are: Bacteroides sp., Prevotella sp., and Parabacteroides sp.
The remaining 5 to 10 percent of the human gut microbiota is made up of four additional bacterial phyla.
3. ACTINOBACTERIA. This phylum contains Bifidobacterium sp.
- Bifidobacterium sp. This genus contains 30 species and represents from 3 to 6 percent of the entire adult gut microbiome. These bacteria are acquired as an infant from breast-feeding. Formula bottle fed babies receive far less colonization with this beneficial bacteria. Bifidobacterium can ferment a far wider variety of prebiotic foods than most other bacteria to produce beneficial postbiotics like short chain fatty acids. Their additional benefits include: regulation of intestinal microbial balance, inhibiting harmful bacteria, modulation of both local and general immune response, repression of pro-cancer enzymatic activity within the microbiota, and production of vitamins.
4. FUSOBACTERIA . This phylum contains Fusobacterium sp,
5. VERRUCOMICROBIA. This phylum contains Akkermansia sp.
- Akkermansia sp. Recently attempts have been made to use newly discovered probiotic species like Akkermansia mucinophila to act like a single species silver bullet to treat specific disease states like metabolic syndrome or obesity, both associated with “leaky gut”. I believe this is unwise considering the extreme complexity and dynamism of each individual’s gut microbiome dysbiosis involvement in these disease states. Besides, simply switching to a plant protein based Mediterranean style diet from a SAD pattern of eating results in naturally increasing the numbers of Akkermansia mucinophila in a balanced and sustainable manner as part of the individual’s core microbiome. Its far less expensive as well. Without change in the diet, the Akkermansia will likely remain just “a tourist” and fail to colonize effectively, requiring ongoing purchase and use of this beneficial probiotic.
6. PROTEOBACTERIA. This phylum contains Escherichia sp. and Shigella sp.
In addition other phyla like BACILLOTA containing Bacillus sp. make up very small percentages of the “normal” gut microbiome. Further, recent advances in metagenomics, a method of reverse engineering the identity of bacteria that cannot be grown in a lab independently outside the body, is revealing more and more new bacterial complexity within the gut microbiome all the time.
The exact number of individual species within each phylum is always dynamic and changing based on both short and long term environmental factors like diet and exercise. And the way in which each phylum’s relative percentage of the whole microbiome is changed by specific species increasing or decreasing within the phylum group, is a much more exacting way of determining what may be a normal range of microbiota for each individual.
How They Live: Biofilms. The close contact of massive numbers of friendly gut microbiota consortiums with the intestinal epithelial cells to create good health is made possible by the creation and stability of bacterial biofilms located in the outer mucus layer of the intestine. But overgrowth of pathogenic bacterial biofilms and their stability is also associated with many diseases.
Biofilms are organized microbial aggregates that live within a matrix created by them. This consists of extracellular polysaccharides (EPS) that are irreversibly attached to a substrate, or, interface, or, onto each other. Permanent, stable microbiota species within the gut microbiome live within biofilms. Isolated, free-floating bacteria are like visiting tourists and do not persist. Biofilms may contain a single species, multiple different species of the same genera (close relatives), or even widely different bacteria from different phyla. The stability of any biofilm depends of the cooperative, symbiotic relationships being established among the individual biofilm members as well as the local intestinal epithelial cells
The intestinal mucus layer serves as vast protective container for biofilms of differing species of bacteria to build their biofilm associations – like a huge number of different individual stores within a much larger mall structure. Mucus contains receptors for bacterial adhesion especially for healthy bacteria like Lactobacillus sp. and Bifidobacterium sp. Bacteria build their biofilm houses by a process known as quorum sensing (QS). When the concentration and numbers of like bacteria reach a certain threshold, bacterial genes are activated to begin producing and releasing intercellular (bacteria to bacteria) peptides and substituted amino acids termed “auto inducers” (AIs). How the bacteria “know” when to switch into this new mode of being is unclear. The AIs then bind to specific corresponding QS receptors on nearby bacterial surfaces at a high density. These receptors then internalize within the bacteria and activate corresponding binding domains of bacterial genes to regulate physiologic functions that individual bacterial cells cannot perform when living outside the biofilm. This quickly leads to an auto-induction feedback loop that promotes the synchronized development of bacterial populations. QS creates a cooperative change in bacterial gene expression and bacterial behaviors acting more like a multi-celled single organism. The transition of bacteria from a free-living state into a poly-cellular population is a complicated and dynamic process that undergoes multiple changes such as cellular reprogramming, variations in expressions of cell surface molecules, the generation of adhesion molecules, virulence factors, and the production of extracellular poly saccharides (EPS).
The concentration of QS molecules within the EPS biofilm matrix housing the bacteria can be 1000 times higher than in environments where isolated bacteria are found. The EPS stabilizes the biofilm within the mucus layer of the intestine and adjacent to the intestinal epithelial cells. The EPS matrix provides many important advantages for the bacterial cells, including:
- absorbing nutrients from surrounding environment to support bacterial growth
- providing physical stability for the bacterial colony
- increasing the ability of bacteria biofilm to adhere to the intestinal surface by resisting physical distortion
- enhancing cells resilience to environmental stress, like attacks from immune cells or antibiotics of pharmaceutical, Paneth cell, or a different bacterial species origin, and
- enhancing the QS and communication within the bacterial colony.
Once formed, the biofilm typically has 4 stages of growth, development and reproduction:
- Initial Attachment to Substrate via intercellular adhesion molecule synthesis.
- Micro-colony Formation and & Increased EPS Secretion.
- Maturation & Increase of QS activity & Release of Complex Carbohydrate Destroying Enzymes.
- Differentiation and Detachment of Individual Biofilm Fragments to New Locations.
The bacterial colony is able to continuously internally regulate their biofilm size by an increase or decrease in QS for EPS production.
Healthy gut microbiota produce a balanced mix of SCFA metabolites (postbiotics) that provide nourishment for the intestinal epithelial cells and act as signaling molecules for the intestinal immune system network that then supports the growth of specific friendly bacterial biofilms. The plasma cells of the gut associated lymphatic tissue release selective secretory IgA (s IgA) into the mucus layer binding to friendly bacteria enhancing their biofilm formation and impeding and weakening pathogenic bacteria, blocking their aggregation attempts and their biofilm formation. Constant communication is occurring between the intestinal epithelial cells and the biofilms to insure homeostasis and protect gut barrier function. The friendly intestinal (probiotic) microbiota biofilms act to maintain a healthy mucus layer, and out compete pathogenic bacteria via QS activities and consuming their nutrients.
Food Influences (Prebiotics): You Are What You Feed Them. (1,11, 12, 20)
The proportions of macronutrients (carbohydrate, fats, proteins), their quantity and quality, along with their co-ingested micronutrients (beneficial plant polyphenols) or harmful contaminants (artificial sweeteners, herbicides, pesticides, etc.) within any given diet will determine the overall health of your gut microbiome and ultimately, you. Your diet determines the degree of diversity as well as the types and percentages of different specific bacterial species that are making a living within your large intestine at any given time. Each bacterial species contributes to the aggregate of unique molecular patterns presented to your intestinal epithelial cells and gut lymphatic tissues as signals that can prompt responses from your intestine that can be either beneficial or harmful to you. This all depends on the current status and quality of your gut microbiota. In addition, the type and amounts of total bacterial metabolic breakdown products (the postbiotics) that are being produced, by your specific microbiota will determine the strength or weakness of your intestine and its barrier function.
The Standard American Diet (SAD). This pattern of eating is low in dietary fiber (both soluble and insoluble), high in saturated fat, high in refined and processed carbohydrates, high in animal protein (especially beef, pork, etc.) and high in artificial food additives. This type of diet markedly and rapidly changes the microbiota quality in both animals and humans. The numbers of known beneficial species of bacteria are decreased and the numbers of known pathogenic species increases. The production and amounts of short chain fatty acids that feed and strengthen intestinal cells is decreased. The SAD causes defects in the protective mucus layer normally created by the intestinal Goblet cells as well as degrades the effectiveness of host defense peptides (HDP) released by the intestinal Paneth cells. This all leads to creation of a leaky gut barrier and generalized inflammation within your body that is associated with a wide variety of diseases.

Vegetarian and Plant Forward Mediterranean Style Diets (see Culinary Medicine Recipes post) are high in both soluble and insoluble fiber, low in refined and processed carbohydrates, low in saturated fats, moderate in monounsaturated and poly unsaturated fats, and high in plant protein along with some occasional fish sourced animal protein. Artificial food additives are avoided. This type of diet increases diversity of the beneficial gut microbiota especially Bifidobacterium sp., a powerful regulator of healthy intestinal barrier function. The production of beneficial short chain fatty acids is increased strengthing the intestinal lining and decreasing inflammation.

Carbohydrates. Soluble Fiber (long chains of individual sugar molecules) is a great food for your gut microbiota because they can do the heavy metabolic lifting. Your gut microbiome has 650 times more digestive enzymes for soluble fiber, unique to it, than you have. Their breakdown of soluble fiber presented to them from your diet results in the fermentation and production of an array of short chain fatty acids that act as food for your intestinal cells, strengthening them. This process also results in greatly increasing the numbers of these “good guy” bacteria that consume soluble fiber for their food. The bottom line here is that soluble fiber consumption has anti-inflammatory and anti aging qualities, and increases your resistance to disease. Insoluble fiber is not able to be fermented or broken down by you or the microbiota and offers no beneficial breakdown products. But, it does improve intestinal motility, stool bulk and prevents constipation.
Protein Sources and Their Associated Fats. Protein consumption in general creates increased microbiome diversity, but the source of the protein, beef & pork, vs. fish, vs. plants, has very different qualitative effects on the gut microbiome. This is not so much because of the different types of proteins, but mainly because of the amounts and types of fats typically associated with the different types of protein food sources. Beef sourced protein is accompanied by increased saturated fats (SF), fish protein is accompanied by omega 3 polyunsaturated fats (omega 3 PUFA) and plant protein is accompanied by lots of non-fat fiber and much lower amounts of fat content overall.
- Animal proteins (beef & pork) are metabolized somewhat by Bacteroides sp. but the amounts of beneficial SCFAs produced are minimal. And some harmful by products like trimethylamine oxide (now linked to increased atherosclerosis) are produced as well.
- Plant Proteins. Alternatively, some microbial species like Bifidobacteria sp. & Akkarmansia sp. can metabolize plant proteins directly, resulting in increase in their numbers and healthy SCFA products.
- High SF Fats in Animal Protein. Beef protein and its much higher SF content verses plant protein, creates increased bile secretion by the liver into the intestine to help emulsify the fat. This is delivered to the colon where it serves to increase the numbers and overgrowth of Bacteroides and pathogenic Clostridia species that displace and decrease the numbers of bacteria like Bifidobacterium and Lactobacillus species.
- Low Fats in Plant Protein. On the other hand, consumption of low fat associated plant proteins and (or) plant based meat alternatives with their high content of associated soluble fiber, decreases the numbers of Bacteroides and pathogenic Clostridia species, and increases the numbers of Bifidobacteria and Lactobacillus species and their production of lots of healthful anti-inflammatory SCFAs.
- High PUFAs in Fish Protein. Finally, fish sourced protein with associated PUFAs, either do not change the quality of the gut microbiota very much, or, can result sometimes in increases in beneficial bacteria like Bifidobacterium and Lactobacillus.
Plant Sourced Phytonutrients & Polyphenols. These are absorbed efficiently in the upper small intestine, but in plant forward diets significant amounts, still reach the microbiota living in the large intestine. They have been shown to increase the numbers of beneficial Bifidobacterium and Lactobacillus species and decrease the numbers of pathogenic bacterial species. In addition, they have been shown to increase microbiome diversity and specifically to decrease the Firmicutes / Bacteroidetes phyla ratios – an anti-obesity effect. In promoting beneficial bacteria, phytonutrients increase SCFA production and serve as anti-inflammatory compounds for the intestine.
Postbiotics: What They Feed You (1, 5, 13)
Postbiotics can be subdivided into either “parabiotics” –fragments of bacterial cell envelopes or pasteurized (killed) whole probiotic bacteria, or “true postbiotics” – the isolated biochemicals produced by living probiotic bacteria (proteins, enzymes, peptides, bactericides, vitamins, neurotransmitters, organic acids like short chain fatty acid metabolites etc.). You may find these distinctions within supplements being sold, but for clarity, I label both of these subdivisions as postbiotics. There is an astonishing number and array of different postbiotics being produced in your body each day by your gut microbiome. Postbiotics produced naturally are generally considered very safe. But postbiotics sold as isolated dietary supplements will probably have their best use in persons with compromised immune systems, providing similar benefit as probiotics without the possible adverse effects of exposure to live probiotic organisms in this specific population. Of all postbiotics, the short chain fatty acids (SCFAs) are the most studied.
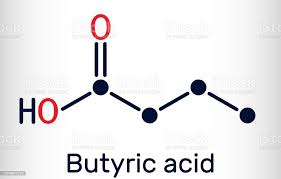
1. SCFAs. These are mainly produced by the digestion and fermentation of soluble fiber from plant based foods by beneficial bacteria. They include: acetate (2 carbons), propionate (3 carbons), butyrate (4 carbons), and valerate (5 carbons). SCFAs have a long and growing list of many important functions within us. Just some of these include: serving as a valuable energy source for gut epithelial cells and the body as a whole, acting as signaling molecules for the gut immune system and stabilizing and balancing its responses, regulation of metabolic set points and thermogenesis by brown fat cells (stabilizing weight), crossing and controlling permeability of the blood brain barrier, optimizing brain microglial function and maturation, and regulating brain neurotransmitters and neuron growth.
2. Other General Postbiotics and Functions.
- Secreted Bacterial Proteins & Peptides preserve barrier function in the intestine, prevent inflammation and have immune modulatory action.
- Aggregation Promoting Factors facilitate colonization of good bacteria and exclusion of pathogens.
- Bacteriocidins mediate inhibitory effects against pathogenic bacteria like Listeria and Pseudomonas.
3. Lactobacillus Species Specific Postbiotics.
- Peptidoglycans serve anti-inflammatory and immune modulation functions
- Teichoic acid acts as an anti-inflammatory.
- Cell wall polysaccharides modulate systemic and mucosal immune responses.
- Cell surface proteins facilitate live Lactobacillus bacterial adhesion to intestinal epithelial cells.
- S layer proteins inhibit adherence and infection of pathogenic bacteria Shigella and E. coli.
- Pili proteins promote the persistence of Lactobacillus strains within the GI track, promote pathogen exclusion and have immune modulatory effects.
- Moonlighting proteins mediate colonization of other probiotic strains and cause competitive displacement of pathogens like Salmonella and Helicobacter pylori.
The receptors for postbiotic signals are concentrated within the GI tract, but are also abundant in distant tissues. These are microbe associated metabolic pattern (MAMP) recognition receptors with a wide variety of mechanistic names such as Toll-like Receptors and G-protein coupled receptors.
How to Create and Maintain a Thriving & Balanced Microbiome Vitality Organ Within You.
Ancient Ayurvedic Medicine understood that each of us has a unique ideal physical constitution known as your Prakruti. Your Vikruti was known as your current state. All disease was thought of as a physiologic situation where the individual’s current state was not properly aligned with their ideal state. The innate intelligence of the body wants to always maintain and return to this place of ideal balance for your specific physical body. Your microbiome “knows” this and is actually quite stable unless it is challenged by adverse conditions. The short list of these adversities is: lack of exercise, poor diet, environmental toxic exposure, stress, and repeated or prolonged antibiotic use. Even inherited conditions that predispose to gut illness like Crohn’s Disease can be mitigated by minimizing these adversities.
- Get Regular Exercise. It’s a powerful balancer of the microbiome.
- Reject the Standard American Diet and Eat a Plant Based Mediterranean Style Diet with lots of soluble fiber, adding Fermented Foods like kimchi, sauerkraut, tempeh etc. Fermented foods contain the trifecta for positive gut health: soluble fiber (prebiotics), live Lactobacillus (probiotic) & lots of Lactobacillus bacterial produced fermentation products like short chain fatty acids (see postbiotics above & posts on this website on Culinary Medicine Recipes & Fasting).
- Stress Reduction. Refuse to overload yourself with too much mental stimulation especially from electronic media. Use meditation, breath work, and spending time out in the natural world to balance your mind, relax, and de-stress yourself.
- Get Quality Sleep (see post on this website on sleep)
- When On Antibiotics Use Pulsed Probiotics and fermented foods to mitigate collateral damage to your microbiome when you have to take antibiotics. (See Post, “Antibiotic Treatment and Your Microbiome: Damage Control & Rehab” on this website ).
References
- Tomova, A et al “The Effects of Vegetarian and Vegan Diets on Gut Microbiota” Frontiers in Nutrition. 6:47 doi 10 3389 (2019)
- Rinninella, E et al “What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Deit and Diseases” Microorganisms. 7: 14 1-22 (2019)
- Wang, H et al “Enteric Neuroimmune Interactions Coordinate Intestinal Responses in Health & Disease” Mucosal Immunity. 15:27 27-39 (2022)
- Seppo, S. et al.; “The Intrnational Scientific Association of Probioytics and Postbiotics (ISAPP) consensus statement on definition & scope of postbiotics”, Nature Reviews – Gastroenterology & Hepatology 18:649 – 664 (2021)
- Silva, Y. et al. “The Role of SCFA from Gut Microbiota in Gut – Brain Communication” Frontiers in Endocrinology. 11:25 pg 1-14 (2020)
- Chen, Y. et al.; “Role & Mechanism of Gut Microbiota in Human Disease” Frontiers in Cellular Microbiology 11: 1-12 (March 2021)
- Worthington, J.J. et al. “Enteroendocrine Cells – Sensory Sentinals of the Intestinal Environment and Orchestrators of Mucosal Immunity” Immunology 11: 1 3-20 (2018)
- Ohad, M et al “Health & Disease Markers Correlate with Gut Microbiome Composition Across Thousands of People” Nature Communications doi.org/10.1038/s41467-020-18871-1 1- 12 (2020)
- Wozniak, D. ; et al. “The Role of Microbiota & Enteroendocrine Cells in Monitoring Homeostasis in the Human Digestive Tract” Advances in Medical sciences. 66: 284-292 (2021)
- Fleming, M; et al. “The Enteric Nervous System and its Emerging Role as a Theraputic Target” Gasteroenterology Research & Practice. https: // doi.org / 10.1155/ 2020/8024171 (2020)
- Peurtolas-Balint, F. et al. ; “Does an Apple a Day Keep the Microbes Away? The Interplay Between Diet, Microbiota, and Host Defense Peptides at the Intestinal Mucousal Barrier” Frontiers in Immunology. 11: 1-17 (2020)
- McBurney, M.I. et al .; “Establisjhing What Constitutes a Healthy Gut Microbiome: State of Science, Regulatoru considerations, and Future Directions” The J. of Nutrition. 149: 1882-1895 (2019)
- Tsegay, T et al: “Paraprobiotics and Postbiotics of Probiotic Lactobacilli, Their Positiv Effects on Host and Action Mechanisms: A Review” Frontiers in Nutrition 7: 1-16 (October 2020)
- Deng, Z. et al “Quorum Sensing”, Biofilm, and Intestinal Mucosal Barrier: Involvement Role of Probiotic” Frontiers in Cellular and Infection Microbiology. 10: 1-10 ( Sept 2020).
- Sanders, M. E.; “Thoughts About Next-generation Probiotics & Postbiotics” Gut Microbiota For Health. pg 1-11 (Dec, 2021)
- .Chelakkot, C et al. “ Mechanisms regulating intestinal barrier integrity and its pathological implications” Experimantal and Molecular Medicine. 50: 103-112 ( 2018)
- Guo et al: “Clostridium species as Probiotics: potentials and challenges” J. of Animal Science and Biotechnology 11:24 1-10 (2020)
- Microbewiki; “Lactobacillus acidophilus” pg 1-6 (2016)
- Microbewiki; “Bifidobacterium” pg 1-4 (2010)
- 0.Beane, et al.; “Effects of Dietary Fibers, Micronutrients and Phytonutrients on Gut Microbiome: A Review” Appl Biol Chem 64:36 1-18 (2021)
- Almeida, A., et al. A unified catalog of 204,938 reference genomes from the human gut microbiome. Nat Biotech. Published online 20 July 2020. DOI: 10.1038/s41587-0
- Liu, D. et al. “Gut and Airway Microbiota and Their Role in COVID-19 Infection and Pathogenesis: A Scoping Review”. Infection doi.org/10.1007/s15010-021-01715-5 (October 2021)