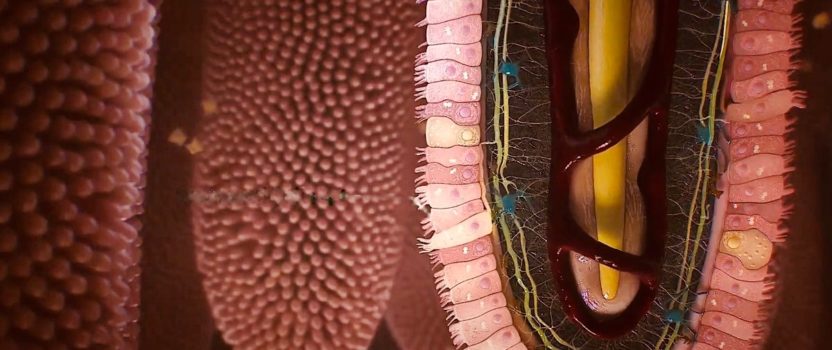
THE FEELING BRAIN: Your Enteric Nervous System (7 March, 2022)
Scott Emerson, MD, ABIHM, FACMT
Timelesshealing
Ancient Wisdom Perspectives. (1)

Thousands of years ago Traditional Chinese Medicine healers and martial artists worked intensively with becoming aware and maximizing of their lower dantian – one of the Daoist three primary reservoirs’ of vital energy in the body. They focused consciousness in this area in order to enhance their physical strength, stamina and achieve robust health. This lower energetic center, located just below the navel, was understood to be the link to the essence of the life force on the Earth. Interestingly, today’s science frontier has discovered a “second brain” in this area of the body, the “gut feeling” intelligence. This “second brain” is orchestrating a constant, intimate, highly complex, cooperative and defensive dance with trillions of the Earth’s earliest life forms.
Overview of the Enteric Nervous System (ENS). (2,3,4)
The ENS is an extensive network of over 500 million neurons embedded as sheathes within the walls of our intestines. This second brain has more than 30 different neurotransmitters and produces 90 % of the body’s serotonin and 50% of the body’s dopamine. It has been shown to act almost independently of the central nervous system. It receives minimal input from the brain, vagus nerve, and the spinal cord. Try to control your growling “stomach” sometime with your mind. The ENS works on its own to coordinate nutrient absorption by intestinal epithelial cells, churning and breaking down food, finely regulating regional intestinal blood flow, expelling waste, and regulating inflammation by communicating with the immediately adjacent, gut associated lymphoid tissue (GALT) – the largest immune organ in our body. It also works to regulate the gut mucus barrier secretion. Like the brain, the ENS has its own support network of neuron focused immune glial cells that provide physical and metabolic support, defend against infection and facilitate nutrient and waste transport for individual neurons.
But the ENS also allows us to “feel”, sense, track, and interact with the inner world of our gut and its extreme complexity and the dynamic state of its contents. This includes hosting 40 trillion bacteria (the microbiome) representing over 1000 different species, co-existing with us in the bioreactor of our large intestine. Our ENS is monitoring, navigating and responding to an environment within in the hollow of our intestine, teeming with a staggering number of other life forms that can be either very beneficial or extremely hostile to us without a finely tuned and shielded regulation. Therefore, it is encased in the wall of the intestine separated by a strict barrier layer of intestinal epithelial cells that act as sensory sentinels, constantly sampling, transducing and sending information about the status of intestinal contents to the local ENS as well as our direct gut to brain connection – the vagus nerve.
The ENS can be weakened by disruptions and imbalances created in this microbiome due to our poor dietary habits, stress, sedentary life style, or antibiotic use. The resulting “dysbiosis” causes a “leaky gut” syndrome, a chronically inflamed state that is associated with many diseases.
FOR A SHORT LIST OF MY RECOMMENDATIONS ON WHAT YOU CAN DO TO MAINTAIN & STRENGTHEN YOUR ENS, SCROLL DOWN TO THE FINAL SECTION OF THIS POST.
The Gut Microbiome: The Non-Us Part of the First & Second Brain. (4, 5, 6, 7, 8)
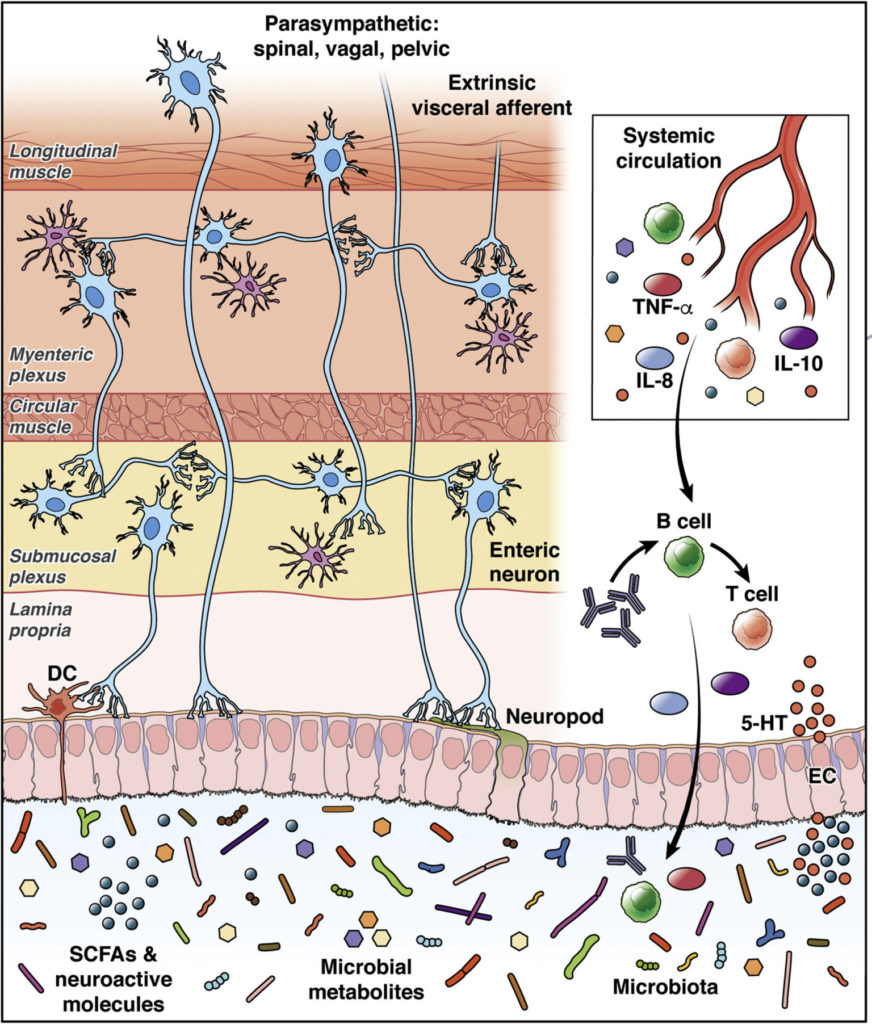
The aggregate of our gut bacteria possesses an astonishing metabolic power, at least equal to the liver, and produces many signaling molecules (postbiotics) that interact with our nervous system via complex mechanisms. They produce a variety of microbe associated molecular patterns (MAMPs), constantly changing in quality and quantity, and continually sensed by the ENS, enteroendocrine cells (EECs) lining the intestine, and the GALT, thus communicating through neural, endocrine and immune signaling channels. This affects the growth and activity of these receiving tissues and triggers release of neurotransmitters, signaling peptides, and cytokines, by these receiving cell types.
1. ENS and vagus (10th cranial) nerve to Microbiome Effects.
Our “second brain” can affect the gut microbiota environment indirectly by altering gut motility, immune modulation, secretion of mucus by intestinal goblet cells as well as antibacterial host defense peptides by intestinal paneth cells.
2. CNS to Microbiome Effects. The CNS via the hypothalamic pituitary adrenal axis can directly decrease gut microbial diversity and increase virulence factors in some enteric pathogenic bacteria through the catecholamine stress response. These CNS stress provoked changes in the quality of gut microbiota have been associated with lowered pain thresholds, anxiety, depression and alteration of metabolic set points.
3. Microbbiome to CNS Effects.
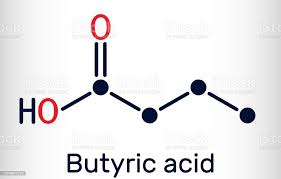
Butyrate. Short chain fatty acids (SCFAs) like butyrate are also produced by gut microbes as metabolic by products (postbiotics) that are released locally and systemically. These act via free fatty acid receptors on the local ENS and distant CNS brain neurons as well as on local EECs and immune cells, affecting their function. Butyrate is actively transported across the blood brain barrier and also acts to strengthen this barrier function. Once in the CNS, butyrate acts to improve the maturity and optimize function of brain microglia support cells as well as improving the brain’s biosynthesis efficiency of important neurotransmitters’, dopamine and norepinephrine. Butyrate stimulates the production of brain derived neurotrophic factor (BDNF), by enhancing DNA histone acetylation within the neuron cell nucleus – an epigenetic mechanism. BDNF then acts to improve neuroplasticity and neuron regeneration in many brain areas. .
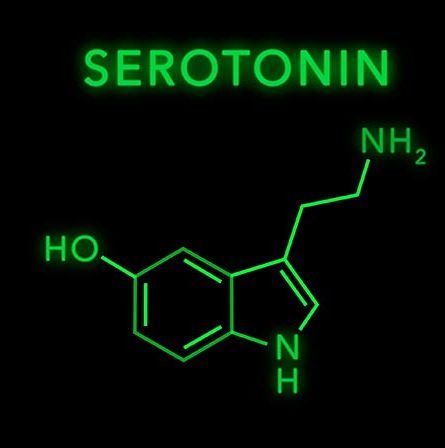
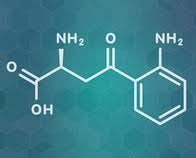
Microbiome / Depression Link. Gut microbes convert dietary tryptophan into lipophilic tryptamine facilitating uptake by EECs, ENS and brain neurons for synthesis of serotonin, a neurotransmitter. Non-pathogenic Clostridia sp., found commonly as part of the “normal” gut microbiota, produce large quantities of SCFA and secondary bile acids (postbiotics) that strongly activate tryptamine hydroxylase enzymes within local EECs, and the brain. This facilitates the synthesis of 5 hydroxy tryptamine (5HT – serotonin) by these cells and neurons. But tryptophan can also be converted and diverted into a metabolic pathway that creates a toxic kynurenine metabolite. Elevated blood levels of kynurenine has been directly associated with brain inflammation, depression and increased risk of suicide. Another beneficial SCFA producing bacteria, Lactobacillus reuteri (probiotic), has also been shown to be able to strongly skew the metabolism of tryptophan toward serotonin synthesis and away from the toxic kynurenine pathway, lowering the blood levels of this compound, and reducing the brain inflammation seen in depressed patients.
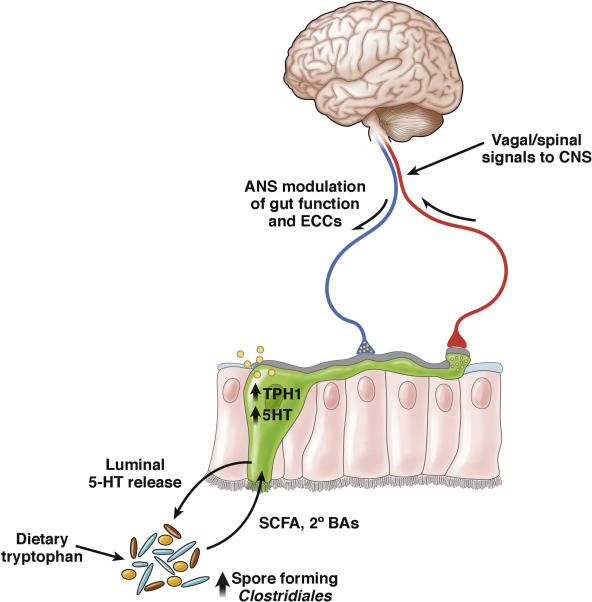
Microbiome Effects on Nervous System Maturation. Finally, the status of your microbiome during the first few years of life may affect normal or abnormal neurological development pathways like the hypothalamic pituitary adrenal axis or the healthy development of the “second brain”. The beneficial microbiota have been shown to have an important role in sculpting the ENS neuroplasticity.
Endoendocrine Cells (EECs): The Non-nerve Part of our Second Brain. (3, 5, 6, 8, 9, 10)
The EECs reside on the inside lining of our intestine. They are secured there at their base to a basement membrane structure made of fibrous proteins. The opposite sides of the cells protrude freely out into the hollow, or lumen part of the intestine. These cells are the primary sensors of the “outside world” contained inside our intestines. They live for 60 days, much longer than other types of intestinal cells. EECs respond to microbial postbiotic molecules and nutrient intestinal content, with complex, multiple, specific, global, and parallel pathways. Their responses target receiving organs, tissues, and cells, both near and far in the body. They do this by manufacturing a wide range of unique peptide neurotransmitters (short connected strands of different amino acid molecules), as well as amine neurotransmitters like serotonin and glutamate. They store these products in designated, separate, intracellular containers (vesicles) located near their basal, attached side. Depending on the type of information that the EECs receive, these intracellular containers may be released into the blood stream for distant effects on the brain, or, just released onto local bacterial populations in the intestinal lumen to affect their activity, or, onto neighboring intestinal epithelial cells and immune cells to direct their function, or, released in the immediate vicinity of primary afferent (outgoing) sensing neuron projections of the enteric nervous system and vagus nerve, stimulating nerve responses.
Recently, and amazingly, it was discovered that although EECs are not neurons, they are capable of growing neuron-like projections termed “neuropods”. These tentacle-like arms contain exceptionally large quantities of both peptide and amine neurotransmitter vesicles out at their ends. These neuropod projections of the EECs can reach out to other individually selected and specifically targeted, intestinal epithelial cells, attaching to them, and releasing their peptides and, or, amine neurotransmitters. This can then direct the specific receiving epithelial cell’s genomic and metabolic activities in specific ways.
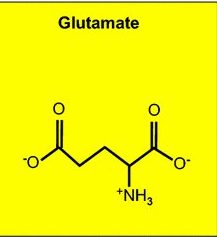
These neuropods are apparently stimulated to grow by known nerve growth factors (neurotrophic factors). They also have the ability to seek out and connect directly to nerve cell terminals. There they can interact and release neurotransmitters, in a synaptic connection with adjacent sensory nerve cells of the ENS (the “second brain”), as well as the vagus nerve’s sensory terminals that input and communicate directly into the first brain’s reward, satiety, pleasure, and behavioral motivation centers. Interestingly, if a glutamate amine neurotransmitter vesicle is released by the neuropod, onto a nerve terminal, an instantaneous emergent signal is generated in the receiving nerve cell. But if a peptide containing neurotransmitter vesicle is released, there is a delayed, slower, less urgent signal response by the receiving nerve cell. And, what does that mean?
All of this is a very nerve-like way for a non-nerve cell to behave. Thus, these very special cells residing in our intestine not only continuously monitor the complex environment of our intestinal contents, but also seem to be able to straddle the divide between an endocrine cell and a nerve cell.
The Enteric Nervous System: The “Second Brain”. (2, 6, 7, 8, 9, 10, 11, 12, 13, 14)
The enteric nervous system (ENS) has a linear tubular structure stretching within the intestinal wall along 28 feet of your bowel. This includes massive aggregates of neuron associations’ known as, a nerve plexus. There are 2 of these. One located nearest the hollow portion of your bowel with projections into the intestinal epithelial cell mass lining the inner intestinal wall – the submucosal plexus. The second group of neurons is located closer to the outer wall of the intestine with major projections into the outer smooth muscle layer of the intestine. These two nerve plexus aggregations are connected to each other by “interneurons” located between the two layers and providing direct connections between the two plexuses.
The ENS communicates with virtually every cell type found within the intestinal tissue. This large nerve network is linked internally and externally by using a dizzying array of different neurotransmitters; like acetylcholine, serotonin, adenosine triphosphate (ATP), various peptides, and even gasses like nitric oxide and carbon monoxide. Beyond neurotransmitters, these nerve cells as well as the gut immune cells also have an ability to directly detect gut microbbiome postbiotic molecular patterns via Toll-like receptors on their cell membranes. This even allows them to distinguish between beneficial and pathologic bacterial signals coming out of the intestinal lumen, and relay this information to the immune system. It can trigger responses like the release of secretory IgA antibodies by gut immune cells out into the mucus layer of the intestinal lumen, ultimately reaching back to either attack or protect specific gut microbes.
The types of specialized ENS cells include:
- Intrinsic Primary Afferent Neurons (IPANS). These are the first sensory nerves to detect the physical, spatial, chemical, nutrient and metabolic state of the intestinal contents.
- Excitatory and Inhibitory Neurons Attached to Smooth Muscle Cells. These nerves receive communication from other neurons and direct and regulate the complex contracting and relaxing movements of the intestinal walls.
- Secretomotor Neurons. These neurons form a circuit with the IPANs. Their main function is to direct the intestinal epithelial cells (IECs) to secrete water into the intestinal lumen.
- Vasomotor Neurons. These work in coordination with the secretomotor neurons to supply proper blood flow to the secretory IECs that are metabolically most active at any given time.
- Interneurons. These are the communication and coordination link between the inner and outer plexus.
- Interstitial Cells of Cajal. These are triangular or stellate shaped cells, described as “primitive neurons” that secrete acetylcholine. They are closely associated with the smooth muscle cells of the outer intestinal wall. They serve as pacemaker cells that receive input from ENS motor neurons linking and coordinating smooth muscle intestinal contractions.
- Enteric Glial Cells. These are the infrastructure support cells for the ENS. They carry a wide array of different receptors and interact with all cell types in the gut wall. They provide repair services for the ENS as well as modulating the totality of its entire synaptic signaling network. They can exist as a mesh-like structure with multiple cells linked together as one giant cell with multiple nuclei. This is known as a syncytium. Continuous communication and cross talk is occurring between these cells and the ENS, as well as the immune cells in the GALT. They control pro-inflammatory / anti-inflammatory set points within the bowel wall that houses the entire ENS and help maintain gut barrier integrity.
Portions of the autonomic nervous system must also be considered as a part of the ENS. The ENS is self-regulating, but does receive some input, but even greater output (“second brain” to “first brain”) connection with the parasympathetic (rest and digest) and sympathetic (fight or flight) autonomic nervous system divisions.
The Parasympathetic Vagus Nerve. The vagus is primarily a sensory nerve that is constantly receiving signals from the ENS. The direction of 90 percent of its nerve impulses are coming from the gut to the brain. This nerve gathers its signals through multiple independent and parallel pathways. It receives direct postbiotic input via molecular pattern recognition Toll-like receptors covering its projecting nerve cell membranes like the ENS nerve cells do. It also innervates the EECs and receives their information via neuropods or paracrine (local hormone) communication using peptide, glutamate or serotonin neurotransmitter specific receptors. The vagus also receives molecular cytokine signals directly from the immune system. Its afferent, receiving pathways directly connect with the neurons of the ENS. But the vagus also receives information from its direct innervation of all of the intestinal epithelial cell types. These communications can bypass all interaction with the ENS, flowing straight to the brain.
The efferent (brain to gut) pathways represent only 10 percent of vagus nerve traffic, but are important in maintaining gut health. They communicate directly with the ENS, but also the IECs and intestinal immune cells using acetylcholine as a neurotransmitter signaler. The vagus directs and maintains the tight junctions via direct stimulus of the acetylcholine nicotinic receptor on IECs. This results in synthesis and export of occludin and zonulin proteins by IECs to maintain tight junction fencing between each cell. In addition, release of acetylcholine by vagal nerve terminals onto goblet cells and paneth cells lining the inner intestinal wall, regulates mucus production and various host defense peptide release by each of these cell types. When the vagus nerve senses gut inflammation it activates a powerful cholinergic anti-inflammatory reflex pathway by releasing its acetylcholine into inflamed areas and onto immune cells, turning off their production of pro-inflammatory cytokines.
The Sympathetic Nervous System. These nerve fibers also interact with all areas of the intestinal wall as well as having direct connections with the ENS. They release norepinephrine as their primary neurotransmitter signaling molecule that stimulates adrenergic receptor target sites. The sympathetic nervous system exerts its effects mainly through activating the adrenergic receptors on glial cells, and directing them to attenuate and repair disruption of IEC barriers. They also stimulate IEC proliferation and growth by stimulating adrenergic receptors on intestinal epithelial stem cells.
Diseases Linked to Abnormal Function of the ENS. (2, 15, 16, 17, 18)
The ENS can be weakened and damaged by chronic inflammation and defects in gut barrier dysfunction (“leaky gut”) causing a variety of gut motility disorders like irritable bowel disease. Rarely, congenital genetic deficiencies in the ENS can lead to severe motility disorders like Hirschsprung’s Disease causing chronic constipation and death from “toxic megacolon” in children.
More recently, pathology beginning within the ENS has been implicated in neurodegenerative diseases like Parkinson’s and Alzheimer’ Disease. There is some evidence that these neurodegenerative diseases result from mis-folded infective protein prions affecting the first brain but actually beginning within the ENS (the “second brain”). It is unclear what may cause a normal protein found in the body to “turn bad”, but chronic inflammation and environmental toxins are leading suspects. Imbalance within the gut microbiota (dysbiosis) is the most common root cause of gut inflammation, and the gut represents a major interface to outside environmental toxin exposure.
These abnormally folded proteins can corrupt other identical normal proteins into their shape, forming inflammatory aggregations within the intestinal nerves. This sets off a pro-inflammatory reaction by enteric glial cells resulting in a “leaky gut” and increased permeability to gut microbes and further inflammation.
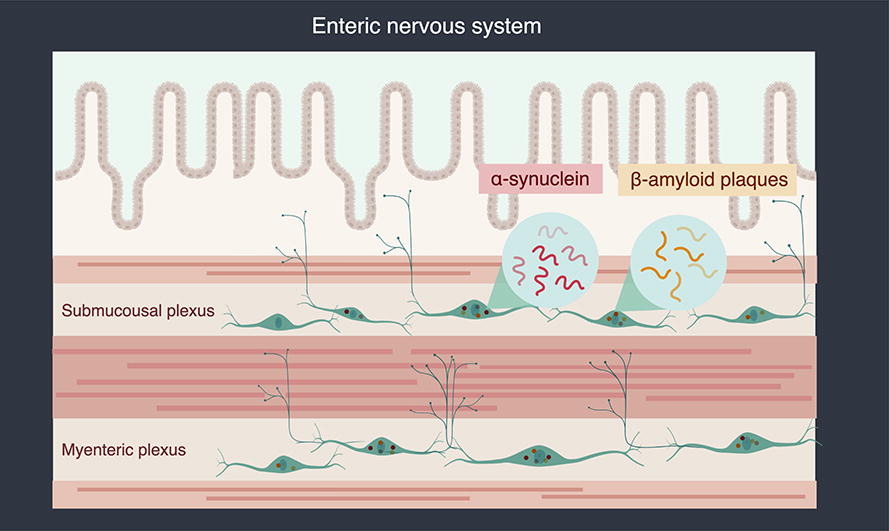
Parkinson’s Disease (PD). Gastrointestinal symptoms like chronic constipation precede the onset of CNS pathologic movement abnormalities in PD by as much as a decade. Mis-folded alpha synuclein proteins recruit normal alpha synuclein into their abnormal shape forming large toxic protein aggregates. These have been found mainly in the secretomotor neurons of the ENC of PD patients. This results in the dysfunction of these neurons. Once these abnormal proteins form, they are not easy for the cell to destroy and they spread from cell to cell within the nerve plexus, ultimately spreading through the vagus nerve into the brain resulting in the classic neuromotor pathology seen in PD. This process can take years.
Alzheimer’s Disease (AD). Although less certain, this same type of process may be occurring in this type of dementia. Abnormal deposits of the beta amyloid protein found in the brains of AD patients have been found within these individual’s intestinal walls, triggering an inflammatory response from the ENS glial cells.
So What Can You Do to Support the Health of Your “Second Brain”? Short Answer – Avoid Inflammation by Maintaining a Diverse, Balanced Gut Microbiome. (19, 20)
- Regular Exercise. Brisk walking an hour a day with an occasional more intense work-out has been shown to correct imbalances and increase the diversity in your gut microbiota very quickly.
- Eat a Plant Forward Diet with Lots of Soluble Fiber & Add Fermented Foods. Fermented foods like kimchi, sauerkraut, and tempeh uniquely offer you the ultimate trifecta for your ENS and gut microbiome: soluble fiber (prebiotics), live Lactobacillus sp. bacteria (probiotics), and lots of bacterial created beneficial short chain fatty acids (postbiotics). And remember black coffee contains 500 mg. of soluble fiber (prebiotic) per cup. (See Culinary Medicine Diet Recipes & Coffee: King of Psyche Delicacies posts on this web site)
- Plant, Work, and Harvest an Organic Garden. Gardening is great outside exercise and exposes you to the great stress reduction and healing power of nature. Plus, a little organic soil in your diet is good for your microbiome health.
- Avoid Collateral Damage to Your Gut Microbiota from Antibiotics. We all need to take antibiotics sometimes for an infection, but there are some simple tools you can use to maintain your balance of beneficial bacteria and gut health while taking them. (See Post, “Antibiotic Treatment and Your Microbiome: Damage Control & Rehab” on this website ).
- Follow the Ancient Wisdom. Work energetically to move your biofield energy and consciousness down into the Lower Dantian – more into the “feeling brain” and less into the “thinking brain” in order to balance the way you are being. Qigong and Taichi are both excellent for this.
References
- Johnson, J.A.; “The Three Dantians” The Institute of integrative Chi Kung & Temple of the Celestial Cloud websites. (2022)
- Fleming, M; et al. “The Enteric Nervous System and its Emerging Role as a Theraputic Target” Gasteroenterology Research & Practice. https: // doi.org / 10.1155/ 2020/8024171 (2020)
- Liddle, R. “Neuropods” Cellular and Molecular Gastroenterology & Hepatology. 7: 739-747 (2019)
- Mohajeri, M.H.; et al. “Relationship Between But Microbiota & Brain Function” Nutrition Reviews. 76 (7): 481-496 (2018)
- Wozniak, D. ; et al. “The Role of Microbiota & Enteroendocrine Cells in Monitoring Homeostasis in the Human Digestive Tract” Advances in Medical sciences. 66: 284-292 (2021)
- Margolis, K. et al “The Microbiota – Gut – Brain Axis: From Motility to Mood” Gastroenterology 160: 1486-1501 (2021)
- Silva, Y. et al. “The Role of SCFA from Gut Microbiota in Gut – Brain Communication” Frontiers in Endocrinology. 11:25 pg 1-14 (2020)
- Martin, C.R. et al. “The Brain – Gut – Microbiome Axis” Cellular & Molecular Gastroenterology Hepatol 6 : 133-148 (2018)
- Worthington, J.J. et al. “Enteroendocrine Cells – Sensory Sentinals of the Intestinal Environment and Orchestrators of Mucosal Immunity” Immunology 11: 1 3-20 (2018)
- Wang, H et al “Enteric Neuroimmune Interactions Coordinate Intestinal Responses in Health & Disease” Mucosal Immunity. 15:27 27-39 (2022)
- Briet, S. et al. “Vagus Nerve as Modulator of Brain – Gut Axis in Psychiatric and Inflamatory Bowel Disorders” Frontiers In Psychiatry. 9: Article 44 (2018)
- Duan, H. et al. “Regulation of Autonomic Nervous System on the Intestine” Frontiers in Physiology. 12: Article 700129 (2021)
- Barajon, I et al “Toll-like Receptors 3,4, and 7 Are Expressed in Enteric Nervous System and Dorsal Root Ganglia” Journal of Histochemistry & Cytochemistry. 57: 11 1013-1023 (2009)
- Ward, S. et al “Interstitial Cells of Cajal Mediate Cholenergic Neurotransmission from Enteric Motor Neurons” J. Neuroscience. 20:4 1393-1403 (2000)
- Benvenuti, L. et al “Enteric Glia at the Crossroads Between Intestinal Immune system & Epithelial Barrier. Implications for Parkinsons Disease” International J. of Molecular Science. 1-16 ( December, 2020)
- Sharkey, K. “Emerging Roles for Enteric Glia in GI Disorders” J Clin Invest. 125 (3) 918-925 (2015)
- Chalazonitis, A. et al “Enteric Nervous System Manifestations of Neurodegenerative Disease” Brain Res. 207-213 (August 2018)
- Rinninella, E et al “What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Deit and Diseases” Microorganisms. 7: 14 1-22 (2019)
- Tomova, A et al “The Effects of Vegetarian and Vegan Diets on Gut Microbiota” Frontiers in Nutrition. 6:47 doi 10 3389 (2019)
- Ohad, M et al “Health & Disease Markers Correlate with Gut Microbiome Composition Across Thousands of People” Nature Communications doi.org/10.1038/s41467-020-18871-1 1- 12 (2020)